The thought of a non-sterile instrument being used during a medical procedure is enough to send chills down any patient’s spine. A simple Google search shows that dirty surgical and diagnostic equipment such as endoscopes remains omnipresent in healthcare and can propose threats to patient safety by potentially increasing risk of surgical site infections (SSIs).
While it’s critical to mitigate any risk of deviation from sterilization processes within a healthcare facility, it’s also important to pay attention to how sterilized instruments are stored and transported afterwards. Sterile packaging systems play an ever-important role in ensuring the sterility of instruments until they reach the next surgical case—yet, they are often overlooked.
Today, there are several options for sterile packaging systems that can be used in the central sterilization (CS) department, including rigid containers, peel pouches of plastic and/or paper, and sterilization wraps (constructed of either woven or nonwoven materials). Each option has its own potential risks to patient safety. Luckily, there’s data available to help healthcare organizations identify these hidden areas of risk within their own CS department and put strategies in place to reduce those risks.
Reviewing the Literature
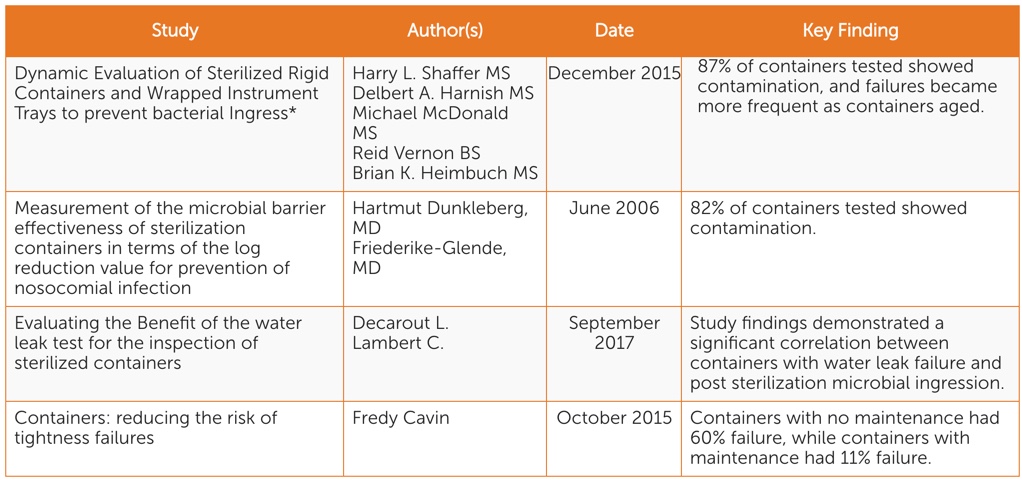
There are four scientific peer-reviewed studies conducted in the past 13 years focusing on identifying and mitigating sterile packaging system-related risks (See Table 1). While each focuses on different aspects of sterility maintenance, all point to the following key considerations:
- When rigid containers are in use, there is potential for instruments to become contaminated during storage and transportation.
- Rigid containers become less effective at maintaining sterility of instruments over time.
- Visual inspection is not enough to identify sterile packaging system sterility risks. The water test is an effective and simple standardized functionality test that can be used in conjunction with visual inspection.
Related Article
Considering Compliance
Potential performance issues inherent with certain types of sterile packaging systems are not the only factor to consider with regard to compliance. Using a mix of sterile packaging systems—which is common—or pressure to meet throughput demand can also impact compliance with a sterile packaging system manufacturer’s instructions for use (IFUs). Anything less than 100 percent compliance with IFUs can lead to improper processing and may impact sterilization packaging performance. By delivering surgical instruments to the OR that haven’t been monitored or processed properly, CS departments can create a false sense of confidence to those who will be utilizing them and potentially put patients at risk.
From Knowledge to Action
It’s impossible to eliminate all risk from sterile processing and the sterile packaging system used along the way, but there are a number of ways healthcare organizations can reduce potential threats to patient safety:
- Ongoing Education: Your sterile packaging system mix may change year-to-year—and with it the corresponding IFUs. Develop a program that both assesses and strengthens user competencies for all sterile packaging systems used within your CS department.
- Thorough Assessment: Perform a facility risk assessment of sterile packaging systems on at least an annual basis and at any equipment or processes change. A thorough risk assessment should take into account average age of container inventory; adherence to IFUs; sterile packaging system integrity and repair; and tear rates.
- Successful Collaboration: Once you have the data and considerations specific to your own organization, share that information far and wide to ensure collaboration on risk mitigation with key partners to the CS department, including surgery and infection prevention.
Protecting patient safety is a shared responsibility across healthcare organizations. While SSIs are on the decline, it remains critical that healthcare professionals continue to identify new potential sources of infection, including sterile packaging systems, and work collaboratively to mitigate those risks.
* Research was funded by Halyard and conducted by Applied Research Associates, an international research laboratory. Halyard did not have any direct involvement in execution of the study. The study took place in an independent laboratory in Panama City, FL, and the study was conducted by independent researchers.
